Emergency Care For Elephants Clinically Ill From Elephant Endotheliotropic Herpes Virus–Haemorrhagic Disease (EEHV-HD)
Fieke Molenaar (ZSL-Whipsnade zoo), Mads Bertelsen and Kathryn Perrin (Copenhagen zoo), Imke Lueders (GEOLifes), Lauren Howard (Houston zoo), Willem Schaftenaar (vet adv. Eur. Elephant TAG) February 9, 2021
(Based on the treatment protocol of Houston Zoo EEHV and the Asian EEHV Working Group)
1. Collect baseline information
- Anamnesis: acitivity pattern, appetite, sleeping pattern.
- Physical examination (body posture, evidence of oedema around eyes, head, neck and ventral abdomen, temperature, blood pressure, changes in color or ulceration of mucous membranes, buccal mucosal bleeding time, etc);
- Blood collection: whole blood (EDTA, heparin and citrate), serum, and blood smear for PCR/qPCR, hematology, WBC (including platelets count!) and chemistry; samples should also be stored for future research, also including antibody titers (please store
any leftover blood collected). - Contact the nearest diagnostic lab that runs PCR and qPCR on all relevant genotypes for emergency diagnosis and arrange sample transport (see address below).
- Blood samples should be tested DAILY using qPCR in order to adjust the treatment regime according to the viral load.
European labs that can perform EEHV qPCR
Date: 9-2-2021
2. Essential sample collection and first treatment may need standing sedation:
- Standing sedation can be performed using detomidine in combination with butorphanol.
- Detomidine 0.01-0.022 mg/kg IM (can be reversed by atipamezole at 3 times the dose of detomidine)AND
- Butorphanol 0.045-0.075 mg/kg given at same time as detomidine. Butorphanol can be reversed with naltrexone at 2.5-5 times the dose of butorphanol in emergency situations, but reversal is not essential and should preferably not be carried out if the calf is considered to be in pain.OR
- Xylazine: 0.04-0.08mg/kg IM (can be reversed with yohimbine or atipamezole)
- Provide supplemental oxygen via nasal cannula whenever possible.
3. Essential supportive fluid therapy:
- Rectal administration of lukewarm, clean water is the first choice of fluid therapy in sick calves. It should be given through a garden hose or rubber tubing after careful removal of fecal balls from the distal part of the rectum (use sufficient lubricant in order to avoid irritation of the rectum mucosa which causes peristaltic activity). When the hose is placed over the horizontal ridge in the rectum (approximately 1 elbow length from the anus), the tube can be advanced for another 100 cm (if possible). A gastric pump can be used; if not available use a large funnel.Rectal fluids should be administered a minimum of 3-4 times per day, up to every 2 hours. A bolus treatment of 10 to 20 ml/kg dose is often used. When finished, the tail should be held down for at least one minute.
- Placement of an intravenous catheter (16-20G IV catheter, with a minimum length of 6 cm to prevent perivascular leaking) in a large, peripheral vein is recommended for:
- Plasma transfusion (supplementation of platelets!) after cross matching recipient blood with donor plasma at 0.5-2 ml/kg BW (see fact box below). The donor should be an adult elephant, preferably PCR-screened on EEHV-viraemia at the time of blood collection.
- Administration of other iv-only medications. Please note that the ear veins are very susceptible to vasculitis, associated with perivascular administration of drugs. Sloughing off of the ear pinna distal to the affected vein is likely in these cases. Extra care should be taken with drugs that are particularly caustic.
- Iv fluid therapy, which will require follow up with rectal fluids.
4. Antiviral drug administration
Antiviral drugs are thought to have an effect during the early stages of viral replication. It is therefore recommended that antiviral theraphy starts as early in the process as is feasible. However treatment may also be attempted in acute cases. The efficacy of the following drugs has not been proven, but all survivor cases have been treated with one or the other of the following drugs:
- Famciclovir: 15 mg/kg orally or rectally TID (grind with mortar and pestle, mix with water to make into a paste and further dilute with water).
- Medications should not be administered rectally within one hour of rectal fluid administration.
- Ganciclovir: in advanced stages of the disease, when a reduced absorption from the intestinal tract can be expected, intravenous administration can be considered more prudent and slow i/v administration of gancivlovir at a dose of 5 mg/kg BID (dissolved in 1 liter of fluid during 1 hour) should be considered.
- Acyclovir: therapeutic doses have yet to be established, but 15mg/kg BID was used in a survivor case: orally, rectally (grind with mortar and pestle, mix with water to make into a paste and further dilute with water) or intravenously.
5. Antibiotic administration
Antibiosis should be considered for treatment of underlying conditions or secondary infections associated with leucopenia and immunosuppression:
- Ceftiofur: 1.1mg/kg IV BID
- Enrofloxacin: 2.5mg/kg PO or rectally SID
- Marbofloxacin: 2mg/kg IV, IM, SQ SID has been used
- Amoxicillin: 11mg/kg IM SID
- Penicillin G: 20,000-50,000 IU/kg IM or IV TID-BID (BID administration has been used in EEHV survivor cases in Asia)
- Any suitable antibiotic with presumed action against invasive gut flora
6. Plasma transfusion
Plasma is currently considered one of the best supportive therapies to provide, as platelets, clotting factors and potentially protective antibodies can thus be provided. Note that the freezing process activates platelets, which renders them useless at the time of transfusion. Therefore – where possible – freshly collected plasma is preferred. The following should be considered for plasma transfusions:
- If frozen plasma is available, this can be given in an early stage of the disease to save time (despite the activated and spent platelets).
- Blood collection from an adult elephant (plasma donor) should be initiated to provide fresh plasma as soon as possible.
- Cross-matching the donor animals with the recipients, especially if one donor will be used on multiple occasions (see text box below).
- PCR screening the donor plasma for current EEHV DNA;
Best practice requires screening of donated plasma for EEHV-DNA by qPCR at the time of collection, prior to administration. Depending on logistical challenges and laboratory availability, there is the possibility that this may delay plasma donation by several hours to two days, which may be detrimental in urgent (severe clinical) cases. If a donor has been screened routinely on a regular basis prior to donation of plasma, this will greatly reduce the risk of introducing more (or different) virus to the calf. It is therefore prudent for collections with at-risk juveniles to identify possible donor elephants (from neighbouring collections if required) and collaborate in advance. - If stored, storage at -80°C is essential;
- Plasma separation does not require a centrifuge. Leaving to stand overnight followed by manual separation (see below) is feasible.
- For administration of plasma, a patent iv cannula and a filtrate infusion giving set are required.
- Dose rate 0.5-2 ml/kg/day – the first 100 ml of each donor should be given slowly to monitor for anaphylaxis.
7. Administration of recombinant Factor VII
May be considered if the elephant has reached the state of hypo-coagulation (blood is not clotting anymore) post-DIC. NovoSeven© has been used in a case at Copenhagen zoo. The drug appeared to improve coagulation, but the calf still died. The drug is prohibitively expensive, but worth further exploration. Suggested dosage: 0.07 mg/kg IV TID, but effect should be monitored using Thrombo Elastography (TEG) or similar.
8. Pain relief
Opioids are a useful adjunct to providing pain relief and in some cases mild sedation to assist in the management of animals being treated. There is the possibility of behavioral changes in the elephant when using opioids and trained behaviors may well be lost or less responsive. A dose of 0.008-0.014 mg/kg butorphanol IM (repeat every 3-4h) is recommended for analgesia.
9. Adjunctive therapies to consider
A single high dose of a short-acting glucocorticosteroid can be considered, as the vascular damage might be caused by an overenthusiastic immune response of the host:
- Flumethasone: 0.005 mg/kg IV or deep IM
- Dexamethasone 0.05-0.5 mg/kg IV or IM
- Triamcinolone 0.067 mg/kg IV (dosage given in 1 survivor in Thailand, Note: This data is under discussion)
10. Anti-inflammatory Drugs:
Although EEHV is thought to be a vasculopathy as opposed to a vasculitis, anti-inflammatories are indicated as part of the analgesic regime as well as reducing secondary inflammation resulting from peripheral edema and hemorrhage. Non-steroidal anti-inflammatories (NSAIDs) may play a useful role in early management of the disease. However it should be noted that in human medicine NSAIDs are contraindicated in cases where peripheral edema or hemorrhagic diathesis is present due to the decreased glomerular filtration rate and the effects on coagulation seen when using NSAIDs. The analgesic and anti-inflammatory effects of these drugs should be weighed against these possible side effects. Flunixin meglumine or other NSAIDS should be administered to patients that appear hydrated or are receiving rectal or IV fluids. Administration of omeprazole for gastrointestinal protection during NSAID treatment should be considered. The equine dose is 0.7 – 1.4 mg/kg PO once daily.
- Flunixin meglumine 0.25 to 0.5 mg/kg IV/IM SID
- Meloxicam 0.2mg/kg IM SID has been used
- Ibuprofen 6mg/kg PO BID
- Phenylbutazone 3mg/kg q48 hours (published dose), 1-2.5mg/kg SID (anecdotal dose) or suxibuzone (loading dose 6 mg/kg/day followed by 3 mg/kg/day)
INTENSIVE CARE OF THE EEHV-HD PATIENT
In any suspected or confirmed EEHV-HD case aggressive supportive therapy and close monitoring of the patient is essential. Rectal administration of fluids (water) is the treatment of first choice. Placement of an intravenous catheter in a large, peripheral vein is recommended for plasma transfusion (supplementation of platelets!) after cross matching recipient blood with donor plasma and administration of other medications. The access to veins should not be jeopardized by unnecessary administration of drugs that can also be administered via another route. If treatment is not possible under training or manual restraint, sedation will be required. Antiviral medication is recommended to reduce or eliminate viral replication and thus reduce the viral load in the patient. Although there is no hard evidence that the antivirals mentioned in this protocol are effective, they are recommended until proven that they don’t work.
Sedatives may be administered to facilitate treatment and to manage pain. Low doses of butorphanol have been safely used in clinical cases. Antibiotics have no effect on viral infections, but must be given to affected animals to prevent and treat underlying and/or secondary infections. If possible, the initial dose should be administered intravenously. Following cessation of intravenous treatment, a change to intramuscular or oral products will be made if appropriate.
Light Sedation in Adult Elephant
- It may be necessary to sedate the dam or other adult herd mates so they are not stressed during manipulations of a calf
- Butorphanol 0.006 mg/kg IM and detomidine 0.0026 mg/kg IM
- Sedation can be reversed as described above but is not necessary
- Alternatively, xylazine or other sedative agents can be used if detomidine is unavailable.
Intravenous Catheter Placement
A temporary IV catheter (16-20 G, minimum 6 cm length) may be placed in the ear, rear leg, or front leg.
Please note that elephants in an intensive care environment can be subject to secondary infections. Attention to hygiene and biosecurity is very important in elephants being treated for EEHV HD, particularly due to their immuno-compromised status.
Intravenous Fluid Therapy
A bolus of IV fluids (0.3 to 1 ml/kg in a calf) can be given to a dehydrated or “shocky” elephant as a resuscitative measure; this bolus could be repeated up to three times with re-evaluation of the patient and vital signs after each bolus. Asian Elephants have very low serum osmolarity and are hyponatraemic and hypochloraemic compared to other species. IV fluids should always be followed with large amounts of rectal fluids (tap water).
Plasma Transfusion
Colloids, such as fresh or frozen plasma or hetastarch are often more effective than crystalloid fluids for volume expansion in viraemic or seriously ill animals. The larger molecules in these fluids do not leak out of capillaries as easily, and increase plasma volume. In this respect, a (fresh!) plasma transfusion has high priority as it provides thrombocytes and coagulation factors. As the preparation of fresh plasma is time consuming, banked plasma can be administered as emergency treatment. To supplement platelets, frozen plasma is NOT suitable, because it contains activated thrombocytes, which will be useless in case of Disseminated Intravascular Coagulopathy like EEHV-HD. The best plasma to administer is so called Platelet Rich Plasma (see below). In addition, plasma from a donor with a high antibody titer may help bind up virus particles in the patient although the role of antibodies is not yet well understood in EEHV-HD. Plasma should only be administered intravenously after (minor) cross-matching donor plasma and recipient whole blood samples to assure compatibility. Additionally, it would be ideal if the donor animal’s blood be PCR tested to ensure the donor does not have a high EEHV viraemia. This information would also be useful as retrospective information. As there will probably be no time for PCR-screening, this can be done later on a stored sample. (Frozen plasma should be checked for the presence of EEHV (PCR) prior to collection or freezing.) The first 100 ml should be given slowly, and heart rate, respiratory rate, and temperature should be monitored. Possible transfusion reactions include fever, rash, or anaphylaxis. Mild signs can be treated by decreasing the rate of transfusion. More severe reactions should be addressed by stopping the transfusion. If no reaction is seen, the transfusion rate can be increased to 0.5-2 ml/kg BW. Clinical improvement may be seen at a plasma dose of 0.5 ml/kg.
Resuming: use banked (frozen) plasma for emergency treatment (coagulation factors, antibodies, colloids) and start preparing for fresh plasma (platelets, coagulation factors, colloids).
Please note that a major cross match needs to be carried out if whole blood is transfused, instead of just plasma.
How to collect platelet rich plasma without specific blood bags:
A. Collect blood in a container with acid citrate dextrose (ACD) as an anticoagulant at the ratio of 6 to 1 and mix gently. In the absence of specific blood bags, empty NaCl-infusion bags or plastic infusion bottles can be used (maintain sterility!) The sample can be kept at room temperature (20-25°C).
B. Instead of ACD, heparin can be added to the donor blood (6,250 IU heparin/liter whole blood)
- Centrifuge at 200G for 10 minutes at room temperature
- Remove plasma and change to a new tube.
- Centrifuge at 1,650G for 10 minutes
- Platelet rich plasma (at bottom of tube) can be kept at 4°C and be used within 5 days.
- If heparin was used as anticoagulant, this can be reversed by protamine HCl
(10 mg protamine HCl/1,000 IU heparin given IV).
Oxygen Therapy
Supplemental oxygen therapy should be administered, when possible, to all patients with clinical signs undergoing treatment for EEHV. Oxygen can be administered at 2-4 l/minute via a flexible tube passed into one side of the trunk. If the elephant will not tolerate oxygen therapy while awake, it may be possible to slip the tube into the trunk while the elephant is sleeping.
EQUIPMENT AND SUPPLIES
The following equipment and supplies will need to be on hand for support during therapy.
Drugs and Equipment Needed:
Placement Of An Intravenous Cannula
Into An Ear Vein In A Juvenile Asian Elephant

In-House Plasma Separation Procedure For Elephants
Design elaborated by Houston Zoo, Inc.
How to make a “Plasma Extractor”
(If you do not have one of these manufactured Plasma Extractors… you can make one!)


Procedure
Step 1:
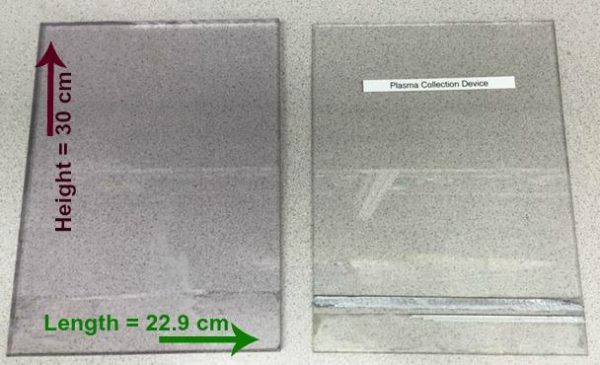
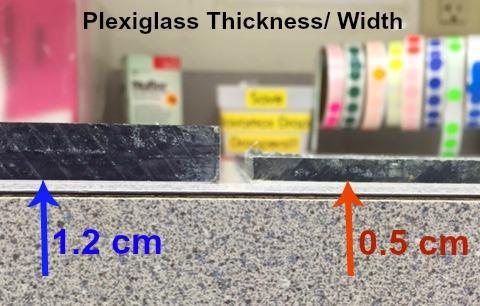
Prepare two pieces of Plexiglas to match the following measurements (note difference
in thickness to provide sturdiness).
- 1st piece: Length= 22.9 cm, Height= 30 cm, Width= 1.2 cm
- 2nd piece: Length= 22.9 cm, Height= 30 cm, Width= 0.5 cm
Step 2:
Align the pieces of Plexiglas together evenly and hold them together. Then wrap duct
tape around the bottom ends of the pieces to keep the Plexiglas together.


Make sure that you can pry the untapped edges apart. The Plexiglas must be able to part wide
enough for a full bag of whole blood to fit in between the pieces.
In-House Plasma Separation Procedure For Elephants
Based on Design elaborated by Houston Zoo, Inc.
Materials
Procedure
- 1Receive bag of whole blood with citrate phosphate dextrose adenine solution (CPDA-1) USP coagulant.
- 2Hang the bag in refrigerator for 6-24 hours to allow for gravitational separation of plasma from red cells. Temperature should be between 0-4 °C. (Figure A)
- 3Carefully remove the blood bag from the refrigerator. Avoid re-suspending the separated red blood cells into the plasma (minimize abrupt motions when handling the collection bag).
- 4Begin plasma separation process by inserting the blood bag into
a.) the “Plasma extractor” or
b.) 2 pieces of Plexiglas duct-taped together. Lay the empty plasma bag beside the extraction apparatus. (Figure B) - 5Break the plastic barrier piece connecting the blood bag to the empty plasma bag. (Figure C)
- 6With one hand, slowly apply gradual pressure to the Plexiglas pieces and with the other hand, use haemostats to hold the connection tubing. The plasma from the blood bag should be flowing into the plasma bag. Be cautious of disrupting the sediment. (Figure D)
- 7When most of the plasma has separated into the plasma bag, quickly clamp off the connecting line with haemostats. Add secondary plastic clamps for extra security. (Figure E)
- 8Using the handheld stripping tool, begin easing the remaining plasma in to the collection bag. (Figure F)
- 9Using another set of haemostats, clamp the line closer to the plasma bag, leaving approximately 30 cm of tubing. Add secondary plastic clamps if necessary. (Figure G)
- 10Cut the connecting line so that the plasma bag separates from the blood bag.
- 11To properly seal the plasma bag for storage, tie 1-3 knots at the open end of the tubing. (Figure H)
- 12Make a loop with the tubing and apply 2-3 evenly spaced metal clips. (Figure I) Slide the first metal clip as close to the bag as possible. Clamp the clips down with the multi-tool. (Figure J)
- 13Weigh the full plasma bag. To determine actual plasma volume, subtract established materials weights (empty plasma bag* and # metal clips**) from the weight of the full plasma bag.
- 14Label the plasma bag with animal ID number, collection date and plasma volume.
- 15Store the plasma in a freezer (preferably -80 °C). However, use fresh plasma for treating EEHV-HD as freezing will activate the thrombocytes, making them useless for EEHV-HD treatment.

FIGURE A
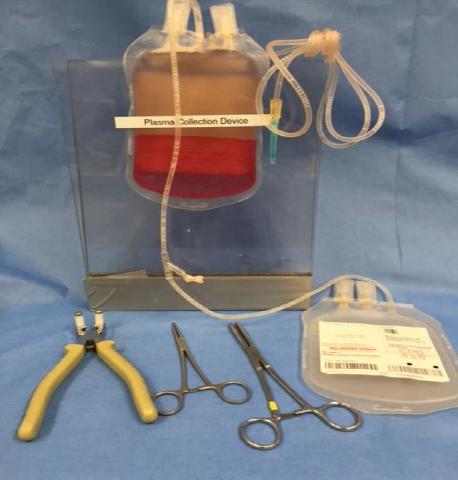
FIGURE B
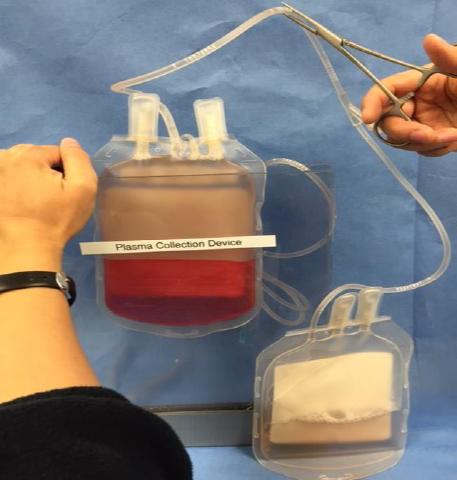
FIGURE D
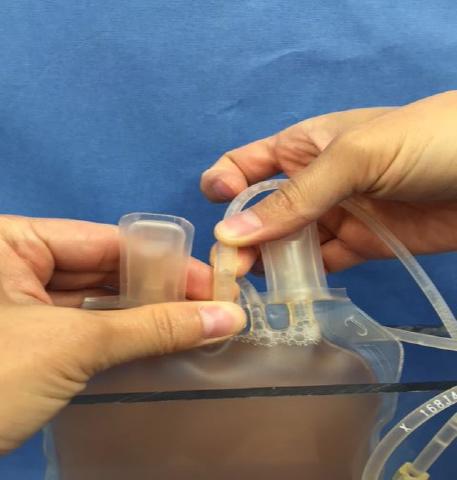
FIGURE C
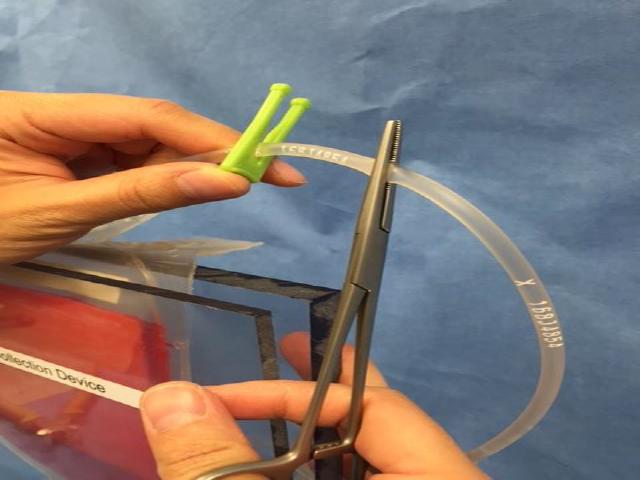
FIGURE E
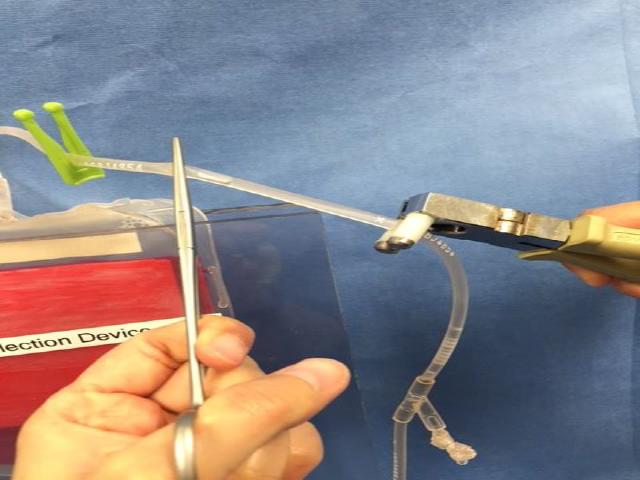
FIGURE F

FIGURE G

FIGURE H
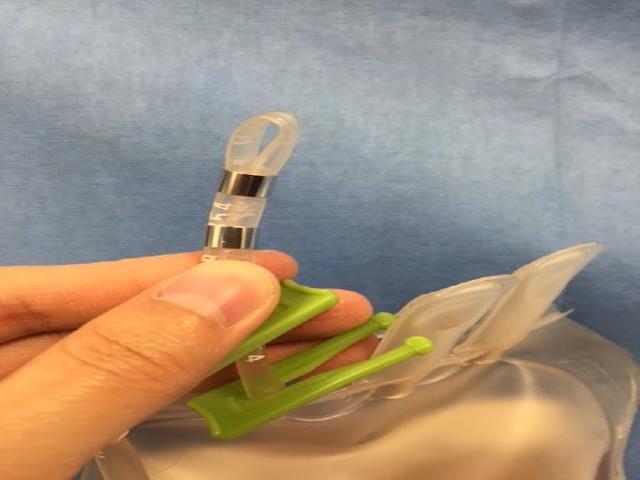
FIGURE I

FIGURE J


